
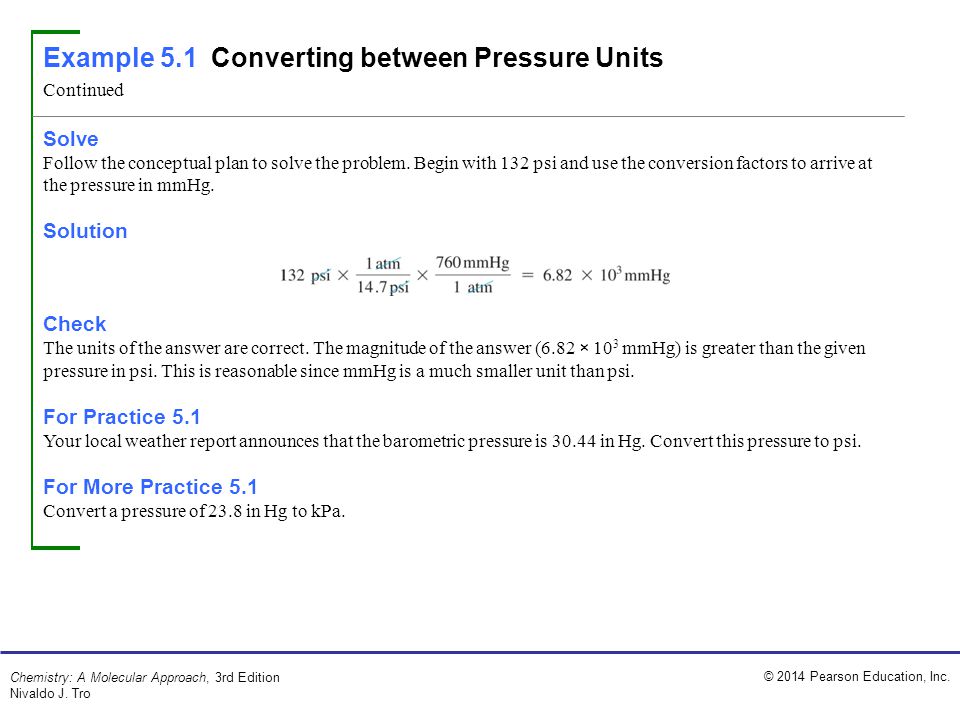
We can use these equivalences as with any equivalences-to perform conversions from one unit to another. We thus have the following equivalences: 1 atm = 76 0 mmHg = 76 0 torr (The torr is named after Evangelista Torricelli, a seventeenth-century Italian scientist who invented the mercury barometer.) With these definitions of pressure, the atmosphere unit is redefined: 1 atm is defined as exactly 760 mmHg, or 760 torr. An equivalent unit is the torr Another name for a millimeter of mercury., which equals 1 mmHg. Unit Conversion Factors Quantity Equivalent Values Mass 1 kg 1000 g 0.001 metric ton 2.20462 lbm 35.27392 oz 1 lbm 16 oz 5 10 4 ton 453.593 g 0.453593 kg Length 1 m 100 cm 1000 mm 106 m 1010 A 1 m 39.37 in 3.2808 ft 1.0936 yd 0.

(mmHg), which is the amount of pressure exerted by a column of mercury exactly 1 mm high. Note that we have Fahrenheit as the biggest unit for length while Per Degree Celsius is the smallest one. Measurement is one of the most fundamental concepts. A more reliable and common unit is millimeters of mercury The amount of pressure exerted by a column of mercury exactly 1 mm high. The formula used to convert atm to Pound-Force per Square Inch is 1 Standard Atmosphere 14.69594877565 Pound-Force per Square Inch. However, “average atmospheric pressure at sea level” is difficult to pinpoint because of atmospheric pressure variations. (atm), which was originally defined as the average atmospheric pressure at sea level. A common unit of pressure is the atmosphere A unit of pressure equal to the average atmospheric pressure at sea level defined as exactly 760 mmHg. However, this is usually too small in magnitude to be useful. The formal, SI-approved unit of pressure is the pascal (Pa), which is defined as 1 N/m 2 (one newton of force over an area of one square meter). The pressure of the atmosphere is about 14.7 pounds of force for every square inch of surface area: 14.7 lb/in 2. Even our atmosphere exerts pressure-in this case, the gas is being “held in” by the earth’s gravity, rather than the gas being in a container. ( P) is defined as the force of all the gas particle/wall collisions divided by the area of the wall: pressure = force areaĪll gases exert pressure it is one of the fundamental measurable quantities of this phase of matter. The accumulation of all these forces distributed over the area of the walls of the container causes something we call pressure. Secondly, how do you convert kPa to mmHg Using the values for each in SI pressure units, any pressure in kilopascals can be converted to millimetre of mercury units using the conversion factor below: 1 mmHg 133.322 Pascals (Pa) Pascals. Although collisions with container walls are elastic (i.e., there is no net energy gain or loss because of the collision), a gas particle does exert a force on the wall during the collision. Solution: multiply the atm value by 760.0 mmHg / atm. The kinetic theory of gases indicates that gas particles are always in motion and are colliding with other particles and the walls of the container holding them.
#Mmhg to atm conversion factor how to


 0 kommentar(er)
0 kommentar(er)
